Surgery Room Procedure
The following guidelines are intended for conducting survival, aseptic rodent surgeries. Training is made available via the Institutional Animal Care and Use Committee (IACUC), and the Animal Resource Facility for those needing it or those required to have it.
I. Area
A. While a dedicated surgical suite is required for larger animals, a separate facility for rodent surgery is not necessary. A room or part of a room that is easily sanitized and not being used for other activities during surgery is appropriate.
B. Specific areas (four) should be designated for animals 1) awaiting surgery, 2) prepping, 3) surgery and 4) recovery. This arrangement helps to prevent cross-contamination of the open incision site.
C. Surgical table and support instruments, e.g. microscopes, head frames, heating blankets, should be cleaned and wiped with a disinfectant such as a quaternary ammonium or chlorhexidine and allowed sufficient contact time (Please contact the ARF for assitance in procuring, if needed.)
II. Surgical Instruments
A. Instruments must be sterilized, preferably using steam (autoclaved). Other acceptable methods of sterilization include: 1) dry heat; 2) gas (ethylene oxide); 3) irradiation; 4) chemical methods (liquid sterilants such as Cidex, Alcide®, Clidox®, etc. (Quaternary ammonium compounds and alcohol are not sterilants). Be sure to follow the guidelines for each method.
B. For multiple surgeries on rodents it is permissible to wash and disinfect instruments between animals if they have been properly sterilized prior to use on the first animal of the session. Wash and disinfect in one of the following manners: 1) immersion in liquid sterilants (Alcide®, Clidox®, etc.) or disinfectant (Chlorhexidine, Quaternary Ammonium, Betadine, Povidone) and rinsed in sterile saline; or 2) Flame sterilize after dipping in 100% alcohol and rinse in sterile saline, or 3) using a hot bead sterilizer.
C. For potentially "dirty" surgeries, e.g. within the gastrointestinal tract, or in the presence of infected wounds, only one surgery should be conducted with each sterile set of instruments.
D. Any fluid or device that contacts the internal environment of the animal should be sterilized by one of the above-mentioned methods.
III. Preparation of the incision site.
A. Shave or pluck the hair liberally.
B. Thoroughly wash with surgical scrub (e.g. Chlorhexidine, Betadine, Povidone). Final wipe should be with alcohol. Allow site to dry. Minimize soaking the body of the rodent as this may lead to irreversible hypothermia and death.
C. Sterile drapes are required for major invasive procedures.
IV. Surgeon
A. The surgeon will wear clean laboratory garments.
B. A new pair of sterile surgical gloves should be used for each surgery if there is substantial risk of contamination. Alternatively, for multiple surgeries, surgeons may wipe their gloves with sterile gauze pads soaked in sterilant in between surgeries, if they have not touched anything outside the sterile field. (see IIB).
C. A surgical mask should be worn during surgical procedures.
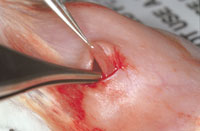
V. Incision Closure
A. Sutures used to close subcutaneous or internal tissues should be absorbable, preferably of low reactivity (e.g., Vicryl, Dexon).
A. Closure of the skin with a non-capillary, non-absorbable material (e.g. nylon, polypropylene or stainless steel) is essential to reduce the risk of postoperative infections. Stainless steel clips (Wound Clips) are the easiest, quickest and also the easiest to remove.
B. All external skin closure suture or staples should be removed within 7-10 days.
VI. Post-surgical Care
A. Animals should be observed to ensure uneventful recovery from anesthesia and surgery.
B. Administer analgesics as required.
C. Provide adequate care for surgical incisions.
D. List animal procedures either on the cage card or in a file maintained in the animal room.
E. In the event that infections or complications ensue, the veterinary staff may review the entire surgical procedure.
VII. General Recommendations
A. Supplemental Heat:
1. For any surgical/anesthesia procedure requiring more than 15 minutes, additional heat should be provided beneath the animal. Circulating hot water heating pads or other devices that offer finite control of the temperature are recommended (Home-use electric heating pads are not recommended). The temperature of such devices should be set at 35-40°C (95-104°F). Settings higher than this may result in thermal-induced necrosis of the skin. Rectal probes, though not essential, are useful to monitor the animal's core body temperature.
2. Supplemental heat should also be provided beneath the recovery cages or in the form of a heat bulb above the cage. The cage should be warmed to no greater than 25°C (85°F). To prevent hyperthermia, animals must be provided a means to migrate away from the heat source once they are awake.
B. Fluids:
Warmed (35-38°C) fluids administered during or after the surgical procedure can hasten recovery and reduce the risk of anesthesia-induced hypothermia and dehydration. 2-5% of the animal's body weight may be given subcutaneously and/or intraperitoneally. Mice should routinely be given 1 ml subcutaneously. Rats should be administered 5-7 ml subcutaneously. Use sterile lactated Ringer's solution or 0.9% saline.
C. Recovery period in the laboratory/surgery area
Animals may be temporarily held in the recovery portion of the designated surgical area for a period not to exceed 24 hours for laboratory mice or rats. For other rodents or animals regulated by the USDA under the AWA, they must be returned to the animal housing facility within 12 hours.
Source: University of New Mexico http://hsc.unm.edu/som/Research/arf/guidelines.shtml#Surgery